The emergence of the concept of biosafety
"Biosafety" has several accepted definitions depending on the discipline involved (veterinary, food, medical or environmental), its linguistic origin or even the country in which it is being used. In Belgium, biosafety is defined as "The safety for human health and the environment, including the protection of biodiversity, during the use of genetically modified organisms or micro-organisms, and during the contained use of pathogenic organisms for humans" (Source: The cooperation agreement of 25 April 1997 between the Federal State and the Regions relating to administrative and scientific coordination in terms of biosafety).
Biosafety therefore makes reference to safety for human health and the environment, genetically modified organisms (or micro-organisms) and pathogenic organisms (a pathogenic organism is a biological agent that can cause disease in immunocompetent humans and poses a risk for individuals directly exposed to it).
The exact moment of origin of biosafety cannot be clearly identified. This discipline has taken shape through different periods in recent history and through different fields (microbiology, molecular biology, veterinary science, guidelines relating to safety, etc). The first steps to this discipline appeared at the time of Pasteur and Koch (around 1890). It was indeed at that time that it appeared necessary to put in place some safety measures in response to the potential risk associated with the exposure to pathogenic micro-organisms. The first infectious diseases acquired in a laboratory were reported at that time. It was another few decades before the notion of a risk to human health linked to the handling of pathogenic micro-organisms was clearly defined. Pioneers such as Sulkin and Pike (Sulkin and Pike, 1949; Sulkin and Pike, 1951) or Collins (Collins CH, Grange JM. The Microbiological Hazards of Occupations. Leeds: Science Reviews. 1990). actively contributed to the implementation of protective measures against biological risks following meticulous investigations carried out in microbiology laboratories.
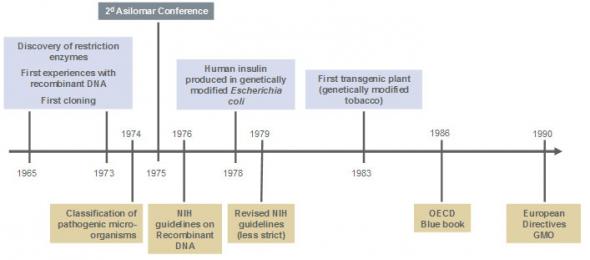
Safety measures in laboratories where pathogenic micro-organisms were handled were firstly implemented in North America and the United Kingdom at the beginning of the 1970s. They included working practices, personnel protection measures and physical containment measures aimed at limiting the spread of biological agents. Safety measures later applied in laboratories handling genetically modified organisms and micro-organisms (GMOs and GMMs) were largely inspired by these guidelines established in microbiology.
In the beginning, biosafety was considered as a sub-discipline of personnel safety (linked to legislation aimed at protecting workers against different types of risks such as chemical or radioactive). However, biological hazard is distinct from other sources of hazard (chemical, radioactive) by the fact that micro-organisms can multiply in vivo (in a host organism) as well as in vitro (in liquid or solid medium).
'Biosafety is based on scientific roots but it is not a science in itself'
Over time biosafety became progressively an entirely separate discipline, partly as a result of two parallel developments: the implementation of an internationally recognised biological risk classification system, and the consequences from the Gordon conference on nucleic acids (1973) and two Asilomar conference (1973 and 1975).
