Since 1986, the Belgian authorities have authorised 168 field trials involving genetically modified (GM) plants. The very first field trial notified in Belgium related to herbicide-tolerant GM tobacco. The last notification related to a GM maize with modified growth characteristics.
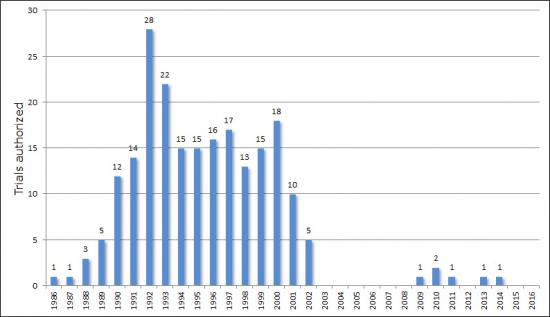
Field trials involving GM plants notified in Belgium (1986-2017)
It is important to stress that each notification relates to a single GMO (case-by-case assessment) and that some dossiers may be the subject of an authorisation valid for several sites and for several years (Directive 90/220/EEC, which was in force in Belgium until 2005, established two types of procedure for experimental release: a standard procedure - for a one-year authorisation - and a simplified procedure making it possible for authorisations extending over a number of years to be issued..
The number of authorisations for the experimental release of GM plants increased between 1986 and 1992 (28 dossiers), then gradually declined until 2003. In terms of surface area, the top was reached in 2000, with a total of 18 field trials underway, covering a total surface area of 110.7 hectares. In 2003, the biotechnology private sector informed the Belgian Government that it would submit no new dossiers until the legislation on the experimental release of GMOs into the environment had been implemented clearly. The sector decided to halt the trials underway (see text box). The entry into force in 2005 of the new Decree transposing Directive 2001/18/EC has done little to change this situation. Very few dossiers have been submitted since then.
A detailed description of each of the field trials (description of the GMO, scientific advice, terms and conditions for authorisation) is available on this website. Information is also available on the European Commission Joint Research Centre (JRC) database, which contains all the applications for deliberate release into the environment within the EU.
The GMO field trials authorised in Belgium mainly relate to oilseed rape, maize, sugar beet and chicory.
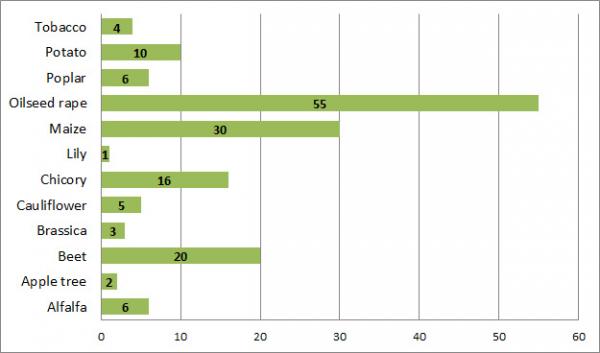
Field trials with GM plants in Belgium – Breakdown by plant species (1986-2017)
The characteristics most commonly tested were male sterility with a view to the development of hybrids and tolerance to glyphosate (Roundup) or glufosinate (Liberty) herbicides. In the last years however only plants with quality traits and pest resistance have been tested.
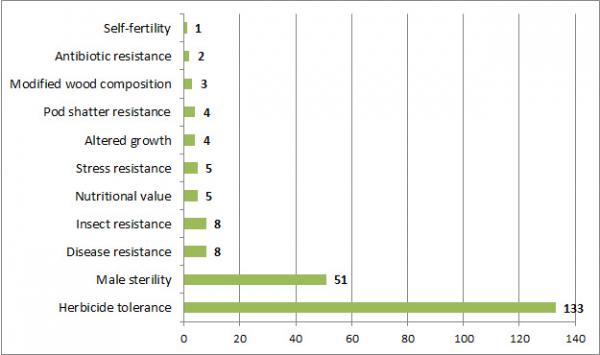
Field trials with GM plants in Belgium – Breakdown by introduced trait (1986-2017)
To summarise, to date, what has primarily been cultivated in Belgium, has been GM oilseed rape. This principally relates to field trials undertaken in the second half of the 1990s and which involved GM lines of the oilseed rape MS8xRF3 (SeedLink hybrid system). These lines were cultivated essentially for purposes of seed production for the development of new varieties, pending European authorisation for the placing on the market of the corresponding GMO.
