- Introduction
- General overview of the biosafety dossier
- Notification forms and user guide
- Procedures
- Contact points
Introduction
All contained use activities involving genetically modified and/or pathogenic organisms must be subject to an environmental declaration or an application for an environmental permit, depending on the risk level associated with the contained use.
The legislation currently in force in Wallonia is the "Arrêté du Gouvernement wallon du 4 juillet 2002 déterminant les conditions sectorielles relatives aux utilisations confinées d'organismes génétiquement modifiés ou pathogènes" (Official Journal 21.09.2002, p. 41711).
=> More information about the regulatory framework
The whole information that has to be given to notify a contained use activity represents the biosafety dossier. In order to facilitate the information and notification procedures and to limit at the minimum the administrative constraints for the notifiers, the SBB has developed notification forms and a user guide, on the basis of the requirements of the regional decree but also of the experience gained of implementing this regulation.
Before any administratve step, the notifier is invited to consult the SBB for any questions about the technical features of the installation and the scientific aspects of the activities. Following this consultation, a certificate of consultation will be given to the notifier by the SBB.
General overview of the biosafety dossier
In Wallonia the biosafety dossier is composed of a technical dossier only. This dossier provides a detailed description of the contained use activity(ies) (including confidential information), the infrastructure, the containment measures, the laboratory practices and any other information allowing the technical expert to assess whether the installations and containment measures comply with the intended contained use.
The technical dossier includes two parts:
- Part 1 corresponding to the form "DONNEES ADMINISTRATIVES". It includes administrative information relating to the overall installation as well as the plans of the installation.
- Part 2 corresponding to the form "INFO OPERATION DOSSIER TECHNIQUE". This form is used to describe in detail any activity of teaching, maintenance of collection, research and development, diagnosis, quality control, production in small and large scale, clinical trial or any other activity performed in the installations.
The technical dossier therefore includes, in addition to part 1, one or several part 2 depending of the number of activities.
The single copy of the technical dossier is sent to the SBB, as Registered Mail, by carrier or by e-mail.
The choice of electronic communication with the SBB as part of the processing of the biosafety dossier must first be confirmed by the notifier using a form determining the modalities: Form for electronic communication (in French).
User guide:
The technical and scientific contents of the forms and the nature of information that is required can sometimes raise questions of interpretation.
A user guide was therefore developed in particular to clarify the use and the interpretation of the forms according to activities of the notifier.
Notification forms and user guide
-
Download the documents :
-
Request them by email to the address contained.use@sciensano.be
In case the contained use notification concerns clinical trials in humans involving clinical research with human cells genetically modified by means of viral vectors, common EU-forms are available on the "advanced therapies" webpage of the EU, which can be used instead of the form "INFO OPERATION DOSSIER TECHNIQUE".
Procedures
Contained uses of GMOs and pathogens are activities mentioned in the list of classified activities subjected to environmental permit (class 1 and 2)* or to declaration (class 3)* (decree of 4/7/2002 defining the list of projects subjected to incidence studies and installations and classified activities - section 73).
* Caution! Do not confuse classes 1, 2 and 3 of the environmental permit with the classes of biological risk of the contained uses.
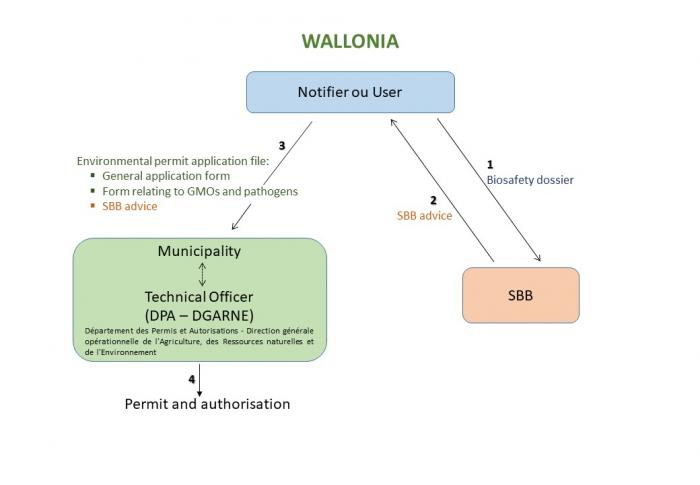
Before any application for an environmental permit, the notifier submits to the advice of the technical expert (the SBB) a biosafety dossier containing in particular a risk assessment of the contained use(s) that take(s) place inside its installation. The SBB send its advice back to the notifier. Afterwards the advice of the SBB will be joined to the request for an environmental permit (Decree of 5/6/2008 amending the Decree of 4/7/2002 related to the procedure and diverse enforcement measures of the Decree of 11 March 1999 concerning the environmental permit).
In Wallonia the competent authority is usually the College of the Mayor and Deputy mayors of the municipality.
In case of contained use of risk class 1
- An environmental permit is not required. The user must make a simple declaration to the municipality. The declaration form includes a GMO section that contains a brief description of the operation, a summary of the risk assessment, the coordinates of the biosafety officer and, if applicable, the biosafety committee and information on waste management. The SBB advice must be attached to the declaration form **.
** Warning: due to the digitalisation of the procedures for request of an environmental permit in Wallonia, the declaration of class 3 installations to the municipality must not contain the advice of the SBB anymore. But according to AGW 05/06/2008, concerning the procedure relating to the Decree of 11/3/1999 on environmental permits, the advice of the SBB on the risk assessment and containment measures is still required and must be added to the declaration form. The procedure is being adapted. - The dossiers for installations subjected to declaration are dealt with by the municipality within 15 to 30 days.
- The contained use can start 15 days after the declaration is submitted, in strict compliance with the operating conditions laid down for this type of activity.
- The declaration has a validity period of maximum 10 years.
In case of contained use of risk class 2, 3 or 4
- An environmental permit is required. The application form should include, in addition to the general annex for the permit, annex 1/19 ("formulaire relatif aux OGM et aux organismes pathogènes"). The annex 1/19 must contain a brief description of the objective, information about the GMOs or pathogens that are handled, an emergency plan project as well as the coordinates of the biosafety officer and, if applicable, the biosafety committee. It is accompanied by the SBB advice.
- The environmental permit dossier is sent to the municipality which is in charge to transmit this request to the administration (DGRNE-DPA which acts as technical civil servant). It issues a synthesis report to the competent authority (the municipality) within 90 to 110 days. It is however necessary to add to this time limit the time needed to acknowledge validity of the notification (20 days).
- The competent authority has a period of 20 to 30 days to notify its decision to the notifier, starting from the receipt of the synthesis report or from the expiry of the deadline for the submission of the synthesis report, if the latter has not been provided within the prescribed period.
- The authorisation for the contained use is issued for a period of maximum 10 years.
- The contained use can start only after the delivery of the authorisation.
Contact points
For any information concerning the practical enforcement of the regulation and the management of dossiers related to the contained use of GMOs and/or pathogens
- SPW ARNE
Département des Permis et Autorisations
Avenue Prince de Liège 15, B-5100 Namur
Tél: 081/33.61.30
http://environnement.wallonie.be
DPA External Directions: for any information concerning the administrative follow-up of dossiers related to the contained use of GMOs and/or pathogens (depending on the localisation of your facility)
-
Direction de Mons
Place du Béguinage 16, B-7000 Mons
Tel: 065/32.82.00 -
Direction de Charleroi
Rue de l'Ecluse 22, B-6000 Charleroi
Tel: 071/65.47.65 -
Direction de Namur
Avenue Reine Astrid 39, B-5000 Namur
Tel: 081/71.53.50 -
Direction de Liège
Espanade Simone Veil 1 (10è étage), B-4000 Liège
Tel: 04/230.39.70
For any information concerning inspections and controls
- SPW ARNE
Département de la Police et des Contrôles
Avenue Prince de Liège 15, B-5100 Namur
Tél: 081/33.61.30
http://environnement.wallonie.be
For any scientific information:
-
Sciensano
Service Biosafety and Biotechnology (SBB)
Rue Juliette Wytsmanstraat 14, B-1050 Brussels
Tel: +32 (0)2 642 52 93
Email: contained.use@sciensano.be
