- Introduction
- General overview of the biosafety dossier
- Notification forms and user guide
- Procedures for first and subsequent uses
- Contacts
Introduction
All contained use activities involving genetically modified and/or pathogenic organisms shall be notified to the regional competent authority. The installations hosting these activities are subject to a preliminary written authorisation in the framework of the environmental permit.
The legislation currently in force in the Brussels-Capital Region is the "Arrêté du 8 novembre 2001 du Gouvernement de la Région de Bruxelles-Capitale relatif à l'utilisation confinée d'organismes génétiquement modifiés et/ou pathogènes et au classement des installations concernées" (MB 26.10.2002, p. 7209).
=> More information about the regulatory framework
This decree foresees that each request for a certificate or for an environmental permit for the above-mentioned installations shall contain a risk assessment of the contained use of GMOs and/or pathogenic organisms as regards risks for human health and the environment, and shall indicate the maximum level of containment that could be reached in the installation. The advice of the Service Biosafety and Biotechnology (SBB) (who acts as technical expert for the Region) is requested during the instruction of requests for certificates and for environmental permits.
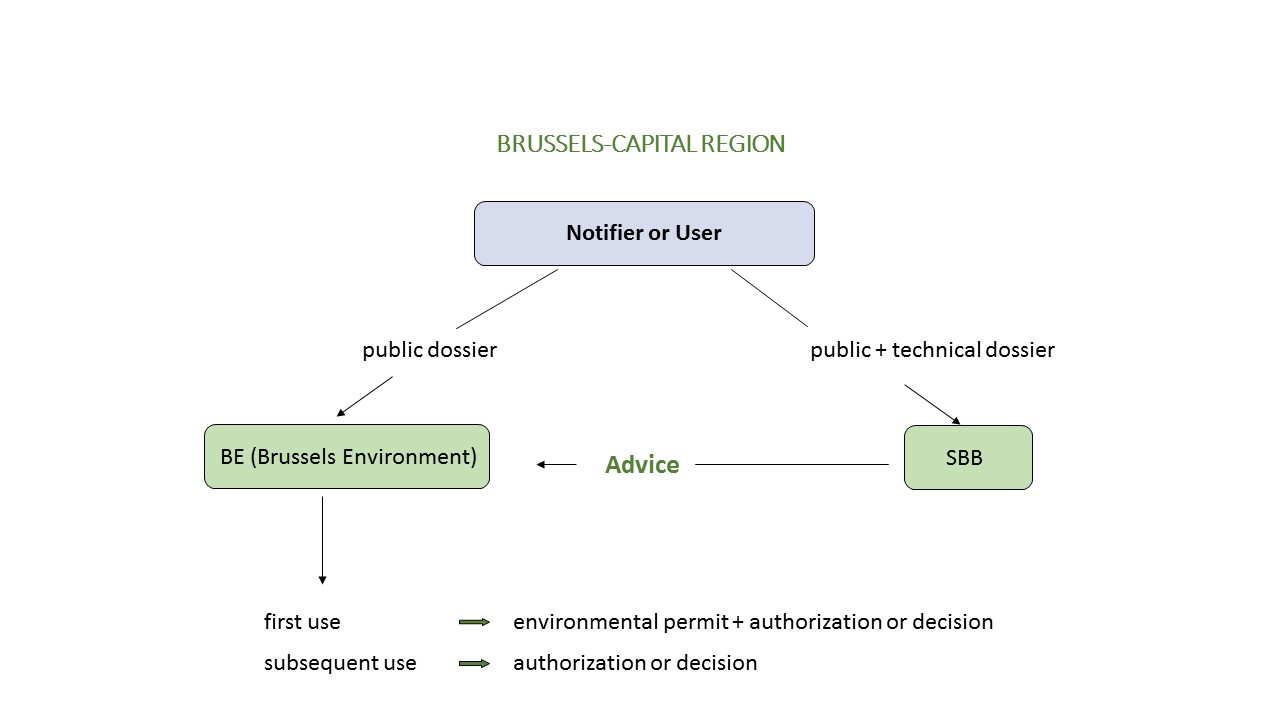
General overview of the biosafety dossier
-
The administrative dossier contains the administrative data related to the facility as well as the plans of the laboratories. It contains the form “ADMINISTRATIVE DATA”.
-
The public dossier is a non-confidential summary of the contained use activities taking place in the facility and can be submitted to public hearing. It includes one or more "INFO OPERATION" public forms (one form per activity).
-
The technical dossier, only intended for the SBB, provides a detailed description of the contained used activities (including confidential information), the infrastructure, the containment measures, the laboratory practices and any other information allowing the technical expert to assess whether the installations and containment measures comply with the intended contained use. It consists of one or more "INFO OPERATION" technical forms (one form per activity).
The administrative dossier, the public dossier and the technical dossier are sent to the SBB (which checks, on behalf of Brussels Environment, the conformity of the information provided in the public and technical dossiers). At the same time, the notifier must also submit a copy of the administrative and public dossier to Brussels Environment (contact details below).
The choice of electronic communication with the SBB as part of the processing of the biosafety dossier must first be confirmed by the notifier using a form determining the modalities: Form for electronic communication in French and in Dutch.
Note on language: since the advice from the SBB and the subsequent authorization from the competent authorities will be written in Dutch or French, the official languages of the Brussels-Capital Region, all the forms are only available in those two languages.
User guide: The technical and scientific contents of the forms and the nature of information that is required can sometimes raise questions of interpretation. A user guide (French and Dutch version) was therefore developed in particular to clarify the use and the interpretation of the forms according to activities of the notifier.
Notification forms and user guide
-
Download the documents :
-
form "ADMINISTRATIVE DATA" (French version - Dutch version)
-
public form "INFO OPERATION" (French version - Dutch version)
-
technical form "INFO OPERATION" (French version - Dutch version)
-
one-time form for electronic communication (French version - Dutch version)
-
User guide (French version - Dutch version)
-
-
Request them by e-mail to the address contained.use@sciensano.be
In case the contained use notification concerns clinical trials in humans involving clinical research with human cells genetically modified by means of viral vectors, common EU-forms are available on the "advanced therapies" webpage of the EU, which can be used instead of the technical form "INFO OPERATION".
Note: The public form “INFO OPERATION” intended for Brussels Environment may be replaced by the technical form “INFO OPERATION”. In that case, the technical form must be completed in one of the official languages of the Region.
Procedures for first and subsequent uses
- No authorisation is required.
- A complete biosafety dossier (public and technical dossiers and administrative dossier) is sent to the SBB. The public and administrative dossiers are also sent to the competent authority (Environment Brussels).
- The SBB sends an advice to the competent authority (Environment Brussels) within 30 days after receipt of the biosafety dossier. If the SBB or the competent authority requests additional information, there is a 'stop the clock' of the procedure.
- The contained use can start the day after the biosafety dossier is submitted and on the condition that the proposed containment measures are applied.
- An authorisation is required.
- A complete biosafety dossier (public and technical dossiers and administrative dossier) is sent to the SBB. The public and administrative dossiers are also sent to the competent authority (Environment Brussels).
- The SBB sends an advice to the competent authority (Environment Brussels) within 30 days after receipt of the biosafety dossier. The competent authority issues a decision within 45 days after receipt of the public dossier addressed to her. If the SBB or the competent authority requests additional information, there is a 'stop the clock' of the procedure.
- The contained use can start 45 days after submission of the dossier to the competent authority.
- The authorisation is generally issued for a period of 5 to 10 years but cannot exceed the date the environmental permit expires.
- An authorisation is required.
- A complete biosafety dossier (public and technical dossiers and administrative dossier) is sent to the SBB. The public and administrative dossiers are also sent to the competent authority (Environment Brussels).
- The SBB sends an advice to the competent authority (Environment Brussels) within 60 days after receipt of the biosafety dossier. The competent authority issues a decision within 90 days after receipt of the public dossier addressed to her. If the SBB or the competent authority requests additional information, there is a 'stop the clock' of the procedure.
- The contained use can start when a written authorisation is issued.
- The authorisation is generally issued for a period of 5 to 10 years but cannot exceed the date the environmental permit expires.
- A technical dossier and an administrative dossier are sent to the SBB only.
- The SBB confirms to the competent authority (Environment Brussels) that the notified contained use is indeed of risk class 1 or alternatively informs the competent authority about any problem associated with the notified risk class.
- The contained use can start the day after the documents are submitted.
- A complete biosafety dossier (public and technical dossiers and administrative dossier) is sent to the SBB. The public and administrative dossiers are also sent to the competent authority (Environment Brussels)..
- The contained use can start the day after the biosafety dossier is submitted on the condition that the installation is already subject to an authorisation and that the containment measures proposed in that first authorisation are applied.
- No new authorisation is needed. However the user can ask for a written authorisation.
- The SBB sends an advice to the competent authority (Environment Brussels) within 30 days after receipt of the biosafety dossier. In the case a written authorisation is asked, the competent authority issues it within 45 days after receipt of the public dossier. If the SBB or the competent authority request additional information, there is a 'stop the clock' of the procedure.
- If an authorisation is issued, it will end the day the environmental permit expires.
- An authorisation is always required.
- A complete biosafety dossier (public and technical dossiers and administrative dossier) is sent to the SBB. The public and administrative dossiers are also sent to the competent authority (Environment Brussels).
- In the case an authorisation has already been issued for a contained use of risk class 3 or 4 in the concerned installation, and the conditions for this previous authorisation are fulfilled, the SBB sends an advice to the competent authority (Environment Brussels) within 30 days after receipt of the biosafety dossier. The competent authority issues a decision within 45 days after receipt of the public dossier. In other cases the SBB sends its advice within 60 days and the competent authority issues a decision within 90 days.
- If the SBB or the competent authority requests additional information, there is a 'stop the clock' of the procedure.
- The contained use can start when a written authorisation is issued.
- The authorisation is generally issued for a period of 5 to 10 years but cannot exceed the date the environmental permit expires.
Contacts
For any information concerning the practical enforcement of the regulation and the management of dossiers related to the contained use of GMOs and/or pathogens
- Environment Brussels
Site de Tour & Taxis
Avenue du Port 86C / 3000
1000 Brussels
Tel.: +32 (0)2 775 75 11 | Fax: +32 (0)2 775 76 21
http://www.environnement.brussels/
For any scientific information:
-
Sciensano
Service Biosafety and Biotechnology (SBB)
Rue Juliette Wytsmanstraat 14, B-1050 Brussels
Tel: +32 (0)2 642 52 93
Email: contained.use@sciensano.be
