In Belgium, the first clinical trial of a medicinal product containing a GMO was notified in 1996. It related to a gene therapy trial involving a recombinant Herpes simplex virus developed for treating cancers. Between 1996 and 2024, a total of 327 clinical trials have been notified, 95% of which concerned humans as the target organism. Overall, 55% of the trials were performed under the “contained use” procedure only, while 43% were done with both the “contained use” and the “deliberate release” procedures, and 2% only under “deliberate release” procedure. The mean number of sites per clinical trial is 2.
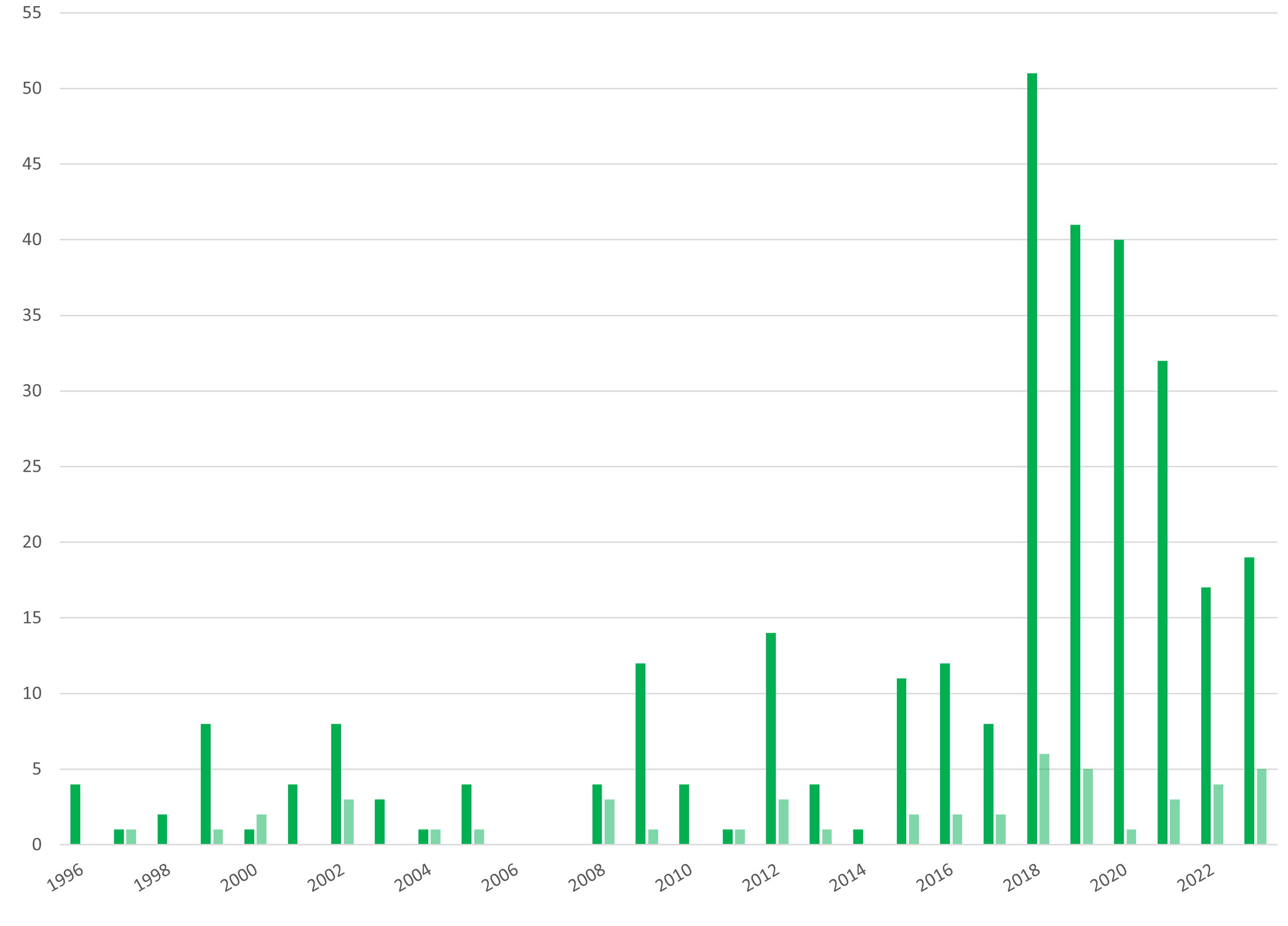
Number of clinical trials in Belgium involving GMO medicinal products for human use, between 1996 and 2024. Dark green is the number of advices delivered for clinical trials under the “contained use” procedure and light green is the number of advices delivered for clinical trials under the “deliberate release” procedure.
The GMO investigational medicinal product (IMP) is a recombinant viral vector in 60% of cases (primarily derived from vaccinia virus, adenovirus and AAV), while GM cells and other GMMs (like bacteria) account for 32% and 8% respectively. The vast majority of these trials relate to treatment of cancer (57%), followed by infectious diseases (27%). Immunotherapy is the most common therapeutic approach (50%), followed by prevention (26%) and gene therapy (18%).
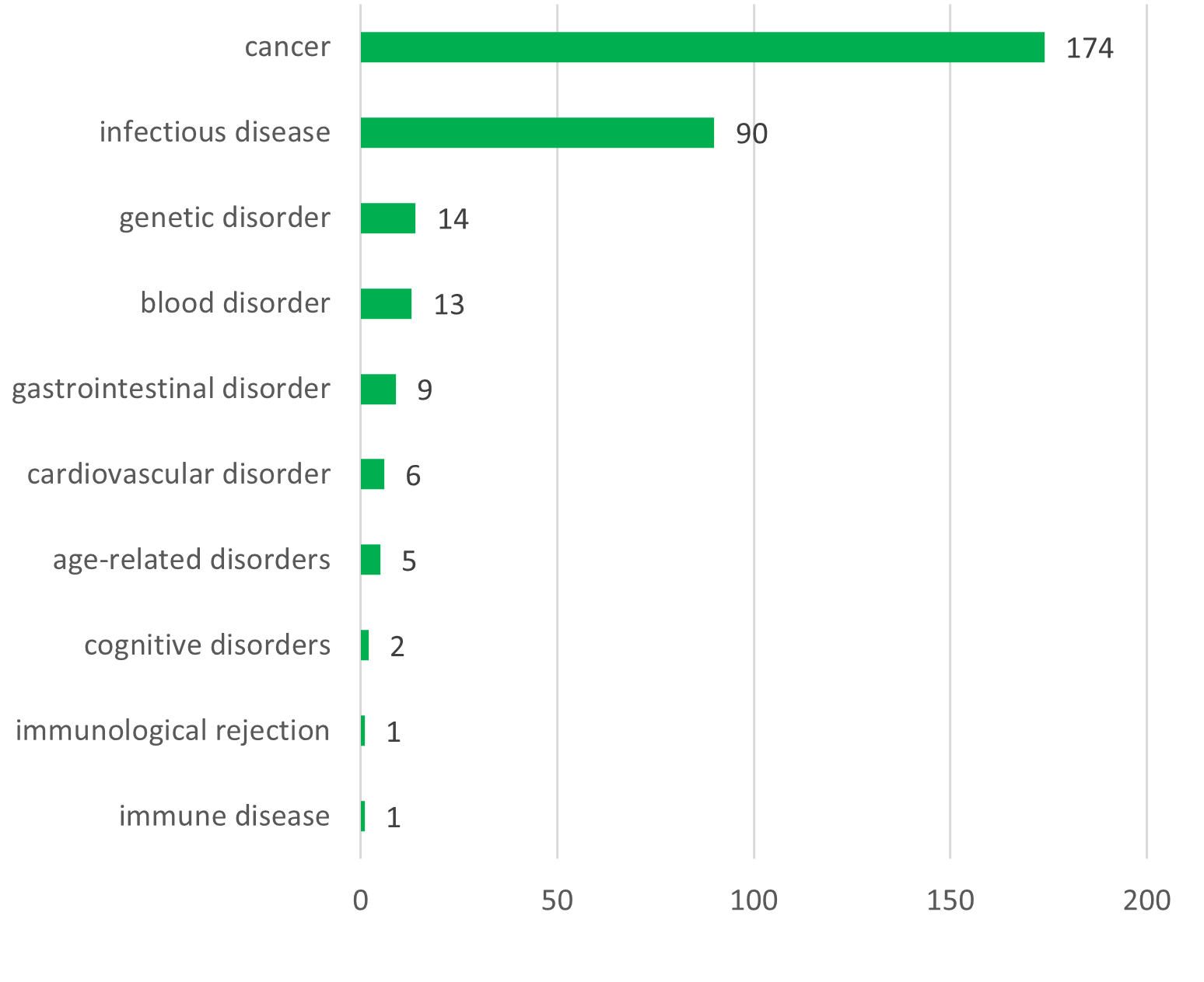
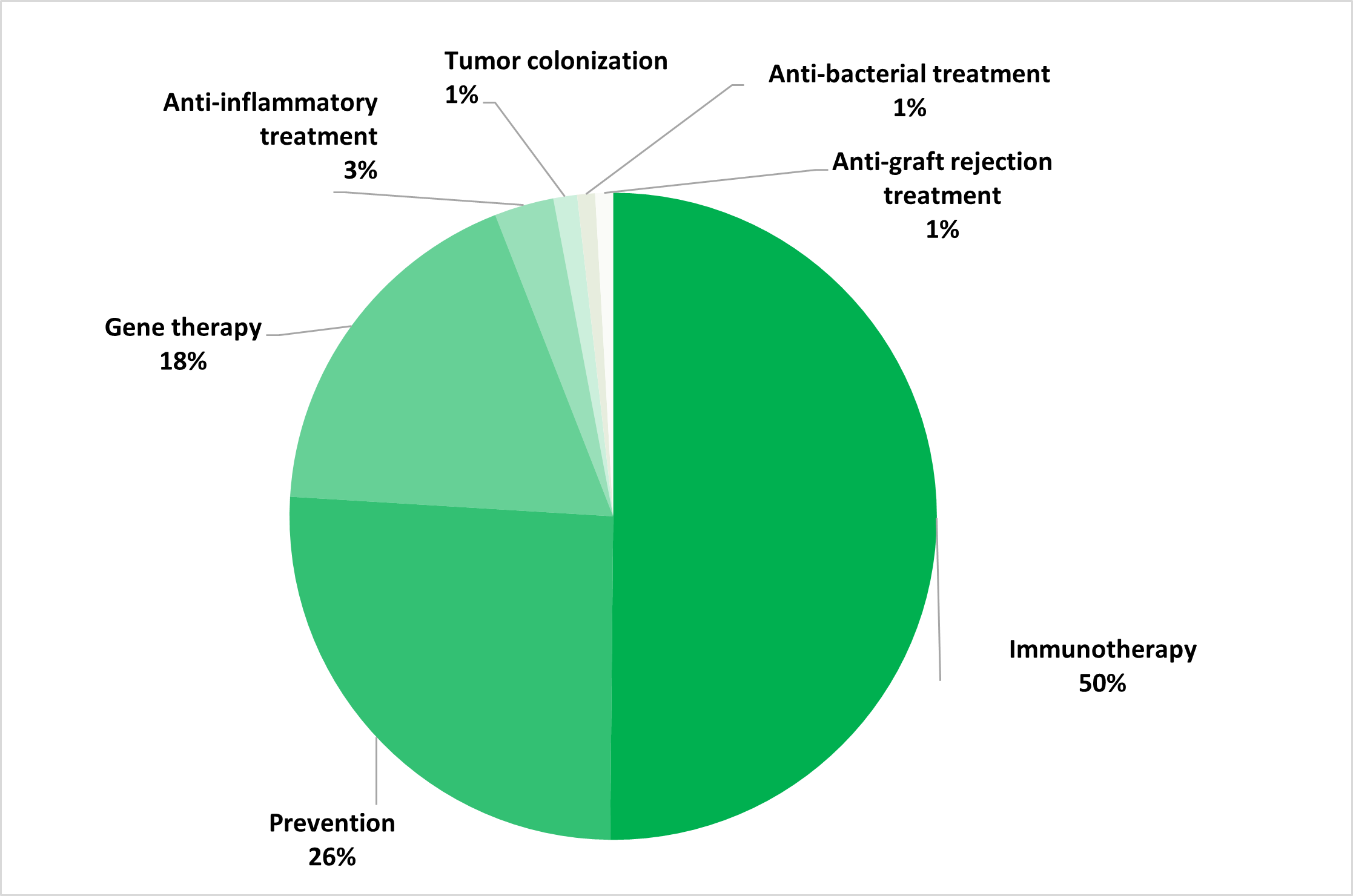
Indications (top) and therapeutic approaches (bottom) for the IMPs used in the clinical trials (N = 337)
A detailed description of each trial (protocol title, trial sponsor, recombinant vector type, authorisation procedure, hospital where the trial took place, investigator's name, etc.) is provided in the database.
