- Introduction
- General overview of the biosafety dossier
- Notification forms, user guide and flowchart
- Procedures for first and subsequent uses
- Contacts
Introduction
All contained use activities involving genetically modified and/or pathogenic organisms shall be reported to the regional competent authority. The installations hosting these activities are subject to a preliminary written authorization in the framework of the environmental permit (for section 51). This permit must be applied for each contained use of genetically modified organisms (GMOs) (section 51.1) and/or pathogenic organisms (section 51.2).
The current legislation in force in the Flemish Region is available on the website Navigator Wetgeving Leefmilieu, Natuur en Energie, with:
- The need for a notification or authorisation, and the fees (hoofdstuk 5 van titel V van het decreet algemene bepalingen milieubeleid (DABM))
- The procedure for notification and authorisation (deel 1 hoofdstuk 1.5 van titel II van het VLAREM)
- De sectorale voorwaarden onder deel 5 hoofdstuk 5.51 van titel II van het VLAREM en zijn bijlagen (5.51.3 tot 5.51.5).
=> More information about the regulatory framework
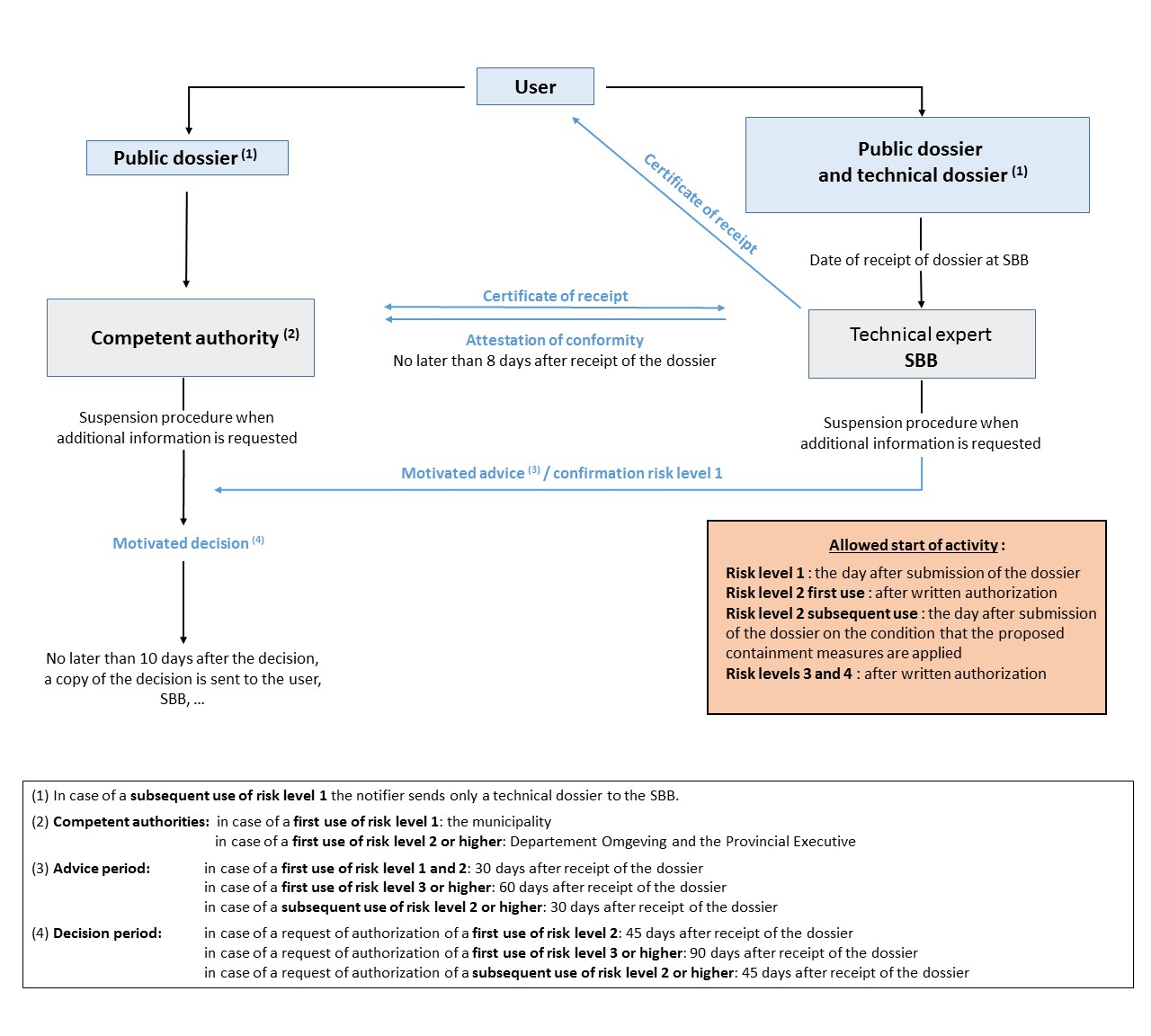
Figure: Schematic representation of the procedures of contained use (click to enlarge)
In order to facilitate the information and notification procedures and to limit at the minimum the administrative constraints for the notifiers, the SBB has, in collaboration with the "Departement Omgeving", developed notification forms and a user guide, on the basis of the requirements of the regional decrees but also of the experience gained of implementing these regulations.
If necessary, a consultation with a technical expert from the Service of Biosafety and Biotechnology (SBB) can also be requested - before starting the administrative procedure - to discuss the technical characteristics of the installation and the scientific aspects of the (planned) contained use. This consultation is informal and free of charge. Following the consultation, the SBB will provide a certificate of consultation to the notifier.
General overview of the biosafety dossier
The biosafety dossier consists of 2 parts, a public and a technical dossier.
The public dossier consists of the part "ADMINISTRATIEVE GEGEVENS" and the part "PUBLIEKE INFO ACTIVITEIT" and is intended for the SBB and the competent authorities (see figure or procedures).
The technical dossier consists of the part "ADMINISTRATIEVE GEGEVENS" and the part "TECHNISCHE INFO ACTIVITEIT " and is only intended for the SBB (see procedures).
(A) The part "ADMINISTRATIEVE GEGEVENS" includes administrative information relating to the overall installation as well as the plans of the installation. It has to be signed (handwritten signature) by the following persons:
- the operator of the installation concerned (the applicant or the holder of the environmental permit)
- the biosafety officer.
The signature relates to the complete dossier !
(B) The part "PUBLIEKE INFO ACTIVITEIT" provides a brief description of each contained use activity carried out in this installation, which contains no confidential information. It must be written in a language accessible to the general public (i.e. language of the region).
One form is filled in per activity.
It has to be signed (handwritten signature is not obliged) by the following persons:
- the biosafety officer,
- the user(s) responsible for the activities.
(C) The part "TECHNISCHE INFO ACTIVITEIT" provides a detailed description of each contained use activity carried out in this installation, including confidential information.
One form is filled in per activity.
It has to be signed (handwritten signature is not obliged) by the following persons:
- the biosafety officer,
- the user(s) responsible for the activities.
For these 3 parts, standard forms have been drawn up by the SBB.
In addition, other forms exist which allows to pass on certain modifications without submitting a complete dossier, such as for:
- a continuation / renewal without modification
- a change in premises of an existing activity
- a non-substantial modification of an existing activity
- a change of operator
- a change of user
- a change of biosafety officer
Both the SBB and the competent authority must be informed. The single copy of the technical dossier is sent to the SBB by e-mail. The public dossier is sent, at the same time as the technical dossier, to the SBB, which checks, on behalf of the competent authority, the conformity of the information provided in the public dossier with that in the technical dossier. At the same time, the notifier also submits this public dossier to the relevant competent authority.
The choice of electronic communication with the SBB as part of the processing of the biosafety dossier must first be confirmed by the notifier using a form determining the modalities.
Note on the language: since the advice from the SBB and the subsequent authorization from the competent authority will be written in Dutch, the official language of the Flemish Region, all the forms are only available in Dutch.
User guide:
The technical and scientific content and the nature of the information required in the forms sometimes raises questions of interpretation. A user guide was therefore developed (in Dutch) to clarify the use and the interpretation of the forms according to the activities of the user.
Flowchart procedures for first and subsequent uses:
The flowchart contains a series of targeted questions in chronological order, making it easy to determine the correct procedure. Additionally, concrete examples are provided to clarify different situations. The footnotes include hyperlinks to the relevant forms, ensuring direct access to the necessary documents for an application.
Notification forms, user guide and flowchart
-
Download the documents :
- form "ADMINISTRATIEVE GEGEVENS"
- form "TECHNISCHE INFO ACTIVITEIT"
- form "PUBLIEKE INFO ACTIVITEIT"
- form "VOORTZETTING/HERNIEUWING ZONDER WIJZIGING" (*)
- form "WIJZIGING IN LOKALEN VAN EEN BESTAANDE ACTIVITEIT" (*)
- form "NIET-SUBSTANTIËLE WIJZIGING"
- form "WIJZIGING VAN EXPLOITANT OF GEBRUIKER"
- form "WIJZIGING VAN BIOVEILIGHEIDSCOÖRDINATOR"
- form for electronic communication
- User guide (pdf file in Dutch)
- Flowchart procedures for first and subsequent uses (pdf file in Dutch)
(*) These forms replace the forms "TECHNISCHE INFO ACTIVITEIT" and "PUBLIEKE INFO ACTIVITEIT" in your dossier.
In case the contained use notification concerns clinical trials in humans involving clinical research with human cells genetically modified by means of viral vectors, common EU-forms are available on the "advanced therapies" webpage of the EU, which can be used instead of the form "TECHNISCHE INFO ACTIVITEIT".
Procedures for first and subsequent uses
The contained use of genetically modified organisms and/or pathogens can only take place in installations that hold a valid environmental permit of class 1 for section 51 (section 51.1.2 or higher for GMOs and section 51.2.1 or higher for pathogens), except for a contained use of risk level 1 whereby a valid environmental permit of class 3 is sufficient.
In addition to the environmental permit, a written authorization or a notification is required for the contained use(s) as such depending on the risk of level of the contained use(s). The authorizations are valid until the end of the environmental permit or for an indefinite period, unless it concerns a R&D activity (often 5 years), while authorizations for a first or a subsequent contained use from risk level 3 are valid for 5 to 10 years depending on the evolving nature of the activity.
The environmental permit and the authorization(s) for the contained use are issued by 2 different authorities. The authorizations are delivered by Departement Omgeving.
In the Flemish Region 2 procedures exist, depending on whether the contained use is either a first or a subsequent use.
First contained use
In the context of the legislation on contained use of genetically modified organisms and/or pathogens, an activity falls under the first contained use procedure when there is:
- a first application for an installation ;
- an increase in the risk level compared to previous authorizations under the same establishment number (environmental permit) ;
- a change in the type of biological material compared to previous authorizations under the same establishment number (environmental permit) ;
- an application for a premise that had not previously been authorized under the required containment level under the same establishment number (environmental permit).
In case of a first contained use of risk level 1 (only GMOs):
- no authorization is required. A simple notification for section 51.1.1 under the existing environmental permit of class 3 to the municipality is sufficient (by a simplified procedure).
- a complete biosafety dossier (public and technical dossier) is sent to the SBB. The public dossier only is also sent to the municipality.
- the SBB sends an advice to the competent authority (Departement Omgeving) within 30 days after receipt of the dossier. If the SBB or the competent authority requests additional information, there is a 'stop the clock' of the procedure.
- the contained use can start the day after the biosafety dossier is submitted and on the condition that the proposed containment measures are applied.
In case of a first contained use of risk level 2:
- both an authorization and an environmental permit of class 1 (for section 51.1.2 for GMOs and/or 51.2.1 for pathogens) are required.
- a complete biosafety dossier (public and technical dossier) is sent to the SBB. The public dossier only is also sent to the competent authority (Departement Omgeving).
- the SBB sends an advice to the competent authority (Departement Omgeving) within 30 days after receipt of the biosafety dossier. The competent authority issues a decision within 45 days after receipt of the public dossier addressed to her. If the SBB or the competent authority requests additional information, there is a 'stop the clock' of the procedure.
- the contained use can start when a written authorization is issued and an environmental permit for section 51 is obtained.
- the authorization is generally issued for a period of 5 to 10 years but cannot exceed the date the environmental permit expires.
In case of a first contained use of risk levels 3 and 4:
- both an authorization and an environmental permit of class 1 (for section 51.1.3 or higher for GMOs and/or 51.2.2 or higher for pathogens) are required.
- a complete biosafety dossier (public and technical dossier) is sent to the SBB. The public dossier only is also sent to the competent authority (Departement Omgeving).
- The SBB sends an advice to the competent authority (Departement Omgeving) within 60 days after receipt of the biosafety dossier. The competent authority issues a decision within 90 days after receipt of the public dossier addressed to her. If the SBB or the competent authority request additional information, there is a 'stop the clock' of the procedure.
- The contained use can start when a written authorization is issued and an environmental permit for section 51 is obtained.
- The authorization is generally issued for a period of 5 to 10 years but cannot exceed the date the environmental permit expires.
Subsequent contained use
A "subsequent contained use" refers to each new contained use or every substantial modification, renewal or continuation of a contained use for which a notification/application for authorization under the same section 51 and risk level (or higher) has already been processed in the frame of the “first use” procedure and where all premises have already been authorized under the required containment level.
In case of a subsequent contained use of risk level 1 (only GMOs):
- a risk evaluation (technical dossier) is sent to the SBB only.
- the SBB confirms to the competent authority (Departement Omgeving) that the notified contained use is indeed of risk level 1 or alternatively informs the competent authority about any problem associated with the notified risk level.
- the contained use can start the day after the risk evaluation is submitted.
In case of a subsequent contained use of risk level 2:
- no authorization is required, except in case it concerns a continuation or renewal of activity or a new activity. However the user can always ask for a written authorization, even if it only concerns a modification of a previously authorized activity.
- a complete biosafety dossier (public and technical dossier) is sent to the SBB. The public dossier only is also sent to the competent authority (Departement Omgeving).
- the SBB sends an advice to the competent authority (Departement Omgeving) within 30 days after receipt of the biosafety dossier. In the case a written authorization is asked, the competent authority issues it within 45 days after receipt of the public dossier. If the SBB or the competent authority request additional information, there is a 'stop the clock' of the procedure.
- the contained use can start the day after the biosafety dossier is submitted and on the condition that the proposed containment measures are applied.
- if an authorization is issued, it will be valid for a specific period. In case of a notification, the validity period of the already authorized activity (that the notification of modification is about) remains.
In case of a contained use of risk level 3 or 4:
- an authorization is always required.
- a complete biosafety dossier (public and technical dossier) is sent to the SBB. The public dossier only is also sent to the competent authority (Departement Omgeving).
- the SBB sends an advice to the competent authority (Department Omgeving) within 30 days after receipt of the biosafety dossier. The competent authority issues a decision within 45 days after receipt of the public dossier. If the SBB or the competent authority request additional information, there is a 'stop the clock' of the procedure.
- the contained use can start when a written authorization is issued.
- if an authorization is issued, it will be valid for a specific period or until the end of the environmental permit.
Contacts
For any information concerning the practical enforcement of the regulation and the management of dossiers related to the contained use of GMOs and/or pathogens
- Departement Omgeving
Afdeling Gebiedsontwikkeling, omgevingsplanning en -projecten
Team Erkenningen
Email: erkenningen.omgeving@vlaanderen.be
http://www.omgeving.vlaanderen.be/
For any information concerning inspections and controls
- Departement Omgeving
Afdeling Handhaving
Email: handhaving.omgeving@vlaanderen.be
For any scientific information:
-
Sciensano
Service Biosafety and Biotechnology (SBB)
Rue Juliette Wytsmanstraat 14, B-1050 Brussels
Tel: +32 (0)2 642 52 93
Email: contained.use@sciensano.be