Pages on the EU regulatory framework concerning:
- Contained use of genetically modified micro-organisms
- Deliberate release of GMOs into the environment according to Directive 2001/18/CE
- GM food and feed
- GM medicinal products for human or veterinary use
- Protection of workers from risks related to exposure to biological agents at work
- Other regulations pertaining to biosafety
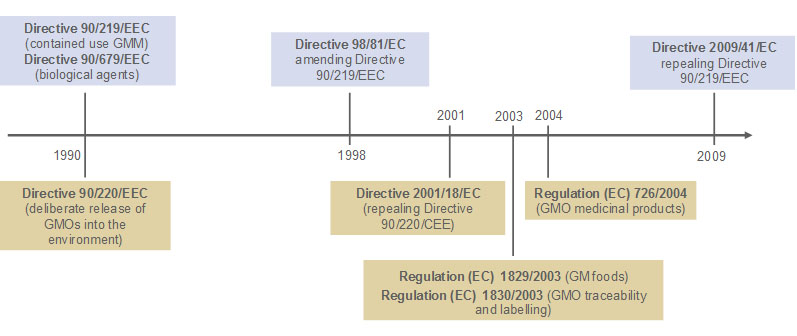
The EU legislation on GMOs has been established in the early 1990s, consisting in 2 legal instruments, Directive 90/219/EEC on the contained use of genetically modified micro-organisms (GMMs) and Directive 90/220/EEC on the deliberate release of genetically modified organisms (GMOs) into the environment. At the same time, Directive 90/679/EEC on the protection of workers from risks related to exposure to biological agents (pathogens) at work was also adopted.
The entire corpus of GMO and biosafety legislation has been amended over the years, leading to the creation of a complex legal framework, including a considerable number of implementing measures to support operation of this framework. The key legal instruments are the “horizontal GMO Directives” 2009/41/EC (contained use of GMMs) and 2001/18/EC (deliberate release of GMOs), and other legislation dealing with specific products, in particular Regulation (EC) 1829/2003 on GMOs destined for food or feed, and Regulation (EC) 726/2004 on medicinal GMOs for human or veterinary use. To ensure a safe use of GMOs, other regulations pertaining to biosafety have also been developped to supplement the legislations mentioned above.