Content
- WHO Global Action Plan for Poliovirus Containment - 4th edition, 2022 : GAP IV
- WHO Global Action Plan - 3rd edition, 2015 : GAP III (Currently replaced by GAP IV)
- GAP III: timeline and global milestones
- Why are the timelines for the containment of poliovirus type 2 prioritised?
- What are the implications of GAP III for organisations, facilities and laboratories and national authorities for the containment of polioviruses?
- What are essential and non-essential facilities?
- What are implications of GAP III for an essential facility handling or storing wild-type polio virus in Belgium?
- What are implications of GAP III for an essential facility handling or storing OPV/Sabin polio virus in Belgium?
- How can a facility in Belgium obtain national containment certification against GAP III?
- Glossary - Polioviruses containment
- Wild poliovirius - Definition and references (opens in a new page)
- Selected references: WHO Poliomyelitis eradication program (opens in a new page)
- Containment of polioviruses worldwide and in Belgium - Historical Background (opens in a new page)
WHO Global Action Plan for Poliovirus Containment - 4th edition, 2022 : GAP IV
The WHO Global Polio Eradication Initiative has made steady progress on the path to eradication. Wild poliovirus type 2 (WPV2) and type 3 (WPV3) have been eradicated globally, in 2015 and 2019 respectively. Today, only type 1 (WPV1) remains circulating in parts of 2 countries. Despite these achievements, the continuing circulation of WPV1 and outbreaks of vaccine-derived polioviruses (cVDPV) present significant obstacles to overcome, before total eradication can be reached. To overcome these obstacles and achieve the goal of a polio-free world, a new strategy has been developed (The Polio Eradication Strategy 2022–2026). To achieve this objective, it is necessary to eradicate WPV1 in endemic countries, to permanently interrupt cVDPD transmission and to prevent cVDPV outbreaks in polio-free regions.
After certification of eradication of all types of WPV and oral poliomyelitis vaccination (OPV) cessation, facilities that will continue to handle poliovirus materials represent the most significant threat to maintaining global eradication.
The updated guidance "WHO Global Action Plan for Poliovirus Containment, 4th edition, 2022 (GAP IV)", which is the chief guidance for poliovirus containment to eliminate any incident in facilities that store and/or work with polioviruses post-eradication, was endorsed by the WHO in 2022. The GAP IV describes the safe handling requirements and community safeguards on the principles of a biorisk management system : the Biorisk Management Standard for Poliovirus-essential Facilities Holding Wild and/or Sabin/OPV Poliovirus Materials. This standard is built on the principles outlined in ISO35001: Biorisk management for laboratories and other related organisations.
The GAP IV came into force on 1 July 2022 and replaces the third edition from 2015 (GAPIII, See below).
WHO Global Action Plan - 3rd edition, 2015 : GAP III (Currently replaced by GAP IV)
In 2015, with the Global Action Plan III (GAP III) the WHO defined a strategy to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses (WPV) and sequential cessation of routine OPV (oral polio vaccine) use.
The long-term goal of GAP III was to ensure that no accidental release of poliovirus type 2 occurs after type-specific eradication. With this aim GAP III provides a framework for facilities who handle and/or store stocks of poliovirus type 2 with due regard for biorisk management (addressing biosafety and biosecurity aspects). GAP III describes a biorisk management system based on 16 elements derived from CWA 15793 (Laboratory Biorisk Management, 2011), addressing areas associated with the design, operation and management for essential facilities (Annexes 2 and 3 of GAP III) and non-essential poliovirus facilities (Annex 6 of GAP III).
GAP III: timeline and global milestones
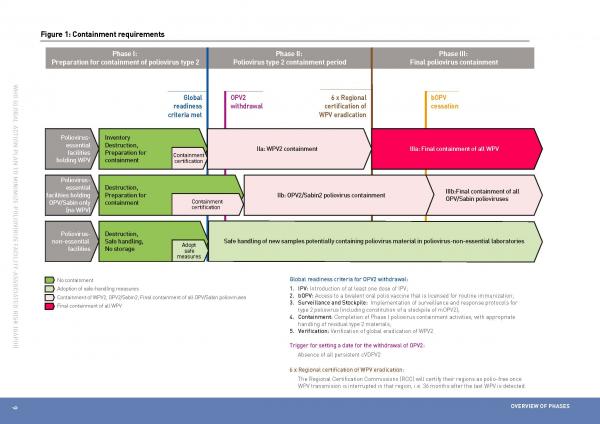
On 25 May 2015, the World Health Assembly (representing all WHO member states) endorsed resolution WHA68.3 entitled ‘Poliomyelitis’, and together with it the GAP III, which outlines a defined timeline and implements three phases linked to global milestones in polio eradication :
- Phase I : Preparation for containment of all type 2 polioviruses (end of 2015*). Phase I is currently in progress and will continue i) for the containment of WPV2 until conditions of global readiness for OPV2 withdrawal have been met; ii) for the containment of OPV2/Sabin2 until 3 months after the switch from trivalent OPV (tOPV: containing type 1, type 2 and type 3) to bivalent OPV (bOPV: containing type 1 and type 3). A trigger for setting a definite date for global OPV2 withdrawal will be the absence of all persistent circulating vaccine-derived polioviruses type 2 (cVDPV2) for at least six months.
- Phase II : Poliovirus Type 2 containment period (2016-2018*): begins for WPV2 materials as soon as criteria for global readiness are met and starts for OPV2/Sabin2 materials within three months of the tOPV-bOPV switch.
- Phase III : Final poliovirus containment (from 2019 onwards*): begins for all WPV when certification of WPV eradication is obtained by all six WHO regions (at least 3 years after the last isolation of WPV) and starts for OPV/Sabin materials three months after global bOPV cessation.
(*) according to current target of GAP III (accessed June, 2015)
Figure 1: GAP III containment requirements, timeline and milestones, from the GAPIII document (PDF)
WHO will release bi-weekly on its Containment of Polioviruses webpages an updated sets of keypoints regarding the containment of the Poliovirus type 2 materials and a map illustrating the reported global progress on the completion of the phase I of GAPIII.
Why are the timelines for the containment of poliovirus type 2 prioritised?
The goal of the sequential cessation of the use of type-specific OPV is to eliminate the risks for vaccine-associated paralytic poliomyelitis (VAPP), outbreaks of circulating vaccine-derived poliovirus (cVDPV) and chronic VDPV infections of immunodeficient persons. The last detection of wild poliovirus type 2 was in 1999. However, in 2013, the type 2 component of trivalent OPV caused more than 90% of vaccine-derived polio viruses (VDPV) and caused approximately 40% of the vaccine-associated paralytic polio cases (VAPP). The risk associated with the use of the type 2 component of trivalent OPV (tOPV contains type 1, type 2 and type 3) thus outweigh the benefits, and it was therefore decided to prioritize and prepare the withdrawal of the type 2 component in oral poliovirus vaccine. While GAP III describes the preparation of all poliovirus containment, it prioritizes the containment of WPV type 2 and the containment of OPV/Sabin PV type 2, in order to be in line with the Polio Eradication and Endgame Strategic Plan 2013-2018. In due regard with the global readiness of OPV2 withdrawal (foreseen in Jan 2016) and the global tOPV-bOPV switch (foreseen in April 2016) and according to timelines set-up by GAP III, facilities intending to work with and store WPV2 or OPV/Sabin PV type 2 in 2016 will be required to be certified nationally against GAP III by the end of 2015.
What are the implications of GAP III for organisations, facilities and laboratories and national authorities for the containment of polioviruses ?
While the aim is to minimize the risk of facility-associated poliomyelitis, it should also be noted that the goal of GAP III is to reduce the number of facilities handling and storing polioviruses. In that context each national authority for the containment of polioviruses needs to assess the reasonability of allowing the continued handling and/or storage of poliovirus in essential facilities on their territory. A distinction is made between essential and non-essential facilities. Non-essential facilities will be encouraged to destroy all unneeded materials and to adopt a non-retention policy while essential facilities will be requested to start certification procedures and to comply with GAP III. Each country will be required to effectively prohibit retention and subsequent acquisition of poliovirus materials in all non-essential facilities.
What are essential and non-essential facilities?
Essential facilities are designated by the Ministry of Health or other designated national body or authority as serving critical national or international functions, that involve handling and storage of needed poliovirus (potentially) infectious materials. Non-essential facilities are facilities that process new specimens that contain or might contain polioviruses and adopt a nonretention policy : these facilities will be required to destroy unneeded PV material or to transfer infectious and potentially infectious WPV materials and OPV/Sabin materials to essential facilities.
A key principle is that at a minimum only those poliovirus facilities that serve critical functions, including IPV and Sabin-IPV production, production and storage of monovalent OPV stockpiles, vaccine quality assurance, diagnostic reagent production, virus diagnostic and reference functions, together with crucial research, would be expected to continue to operate in view of reducing the number of essential facilities worldwide and minimizing the risk of unauthorized release of poliovirus post-eradication.
What are implications of GAP III for an essential facility handling or storing wild-type polio virus in Belgium?
Any facility currently holding wild-type polio virus should already have an authorization according to the regulatory provisions dealing with the contained use of pathogenic organisms. In accordance with timelines and endeavours of the revised GAP III, facilities are now strongly encouraged to destroy all unneeded material or transfer wild-type polio virus to an essential facility.
Should the activities performed within the facility be considered as crucial, thereby implying the need to store WPV, it may be considered as an ‘essential facility’ provided that the facility has been designated by the Minister of Social Affairs and Public Health and that national certification can be obtained. The containment of polioviruses in essential poliovirus facilities will be overseen by and under the responsibility of the National authorities and the National Committee for the Eradication of Poliomyelitis**, who themselves are overseen by the WHO Regional Certification Commission. According to GAP III, once Phase II starts, facilities that have not received national certification will no longer be allowed to handle and/or store WPV2 materials.
**National Committee for the Certification of the Eradication of Poliomyelitis, which is responsible for certifying to the Regional Certification Commission that eradication has been achieved throughout the country.
What are implications of GAP III for an essential facility handling or storing OPV/Sabin polio virus in Belgium ?
Contrary to the containment of wild-type of polioviruses, facilities currently holding Sabin polio virus do not fall under the provisions of the regional decrees regulating the contained use of pathogenic organisms or genetically modified organisms. However, in accordance with timelines and endeavours of the revised GAP III, facilities are now strongly encouraged to destroy all unneeded material or transfer Sabin polio virus to an essential facility.
Should the activities performed within the facility be considered as crucial, thereby implying the need to handle and/or store Sabin PV, it may be considered as an ‘essential facility’ provided that the facility has been designated by the Minister of Social Affairs and Public Health and that national certification can be obtained. The containment of polioviruses in essential poliovirus facilities will be overseen by and under the responsibility of national authorities and the National Committee for the Eradication of Poliomyelitis**, who themselves are overseen by the WHO Regional Certification Commission.
**National Committee for the Certification of the Eradication of Poliomyelitis, which is responsible for certifying to the Regional Certification Commission that eradication has been achieved throughout the country.
How can a facility in Belgium obtain national containment certification against GAP III ?
As GAP III requirements encompass biosafety, biosecurity and management aspects, the set-up of a containment certification procedure will involve several skills and expertise. Timelines presented in GAP III also involve practical challenges for the implementation and certification of GAP III, both for candidate essential facilities and for the national authorities of containment (NAC). The latter will be responsible for issuance of containment certification, endorsed by the Regional Certification Commission (RCC), provided that facilities are adequately assessed and comply with the GAP III requirements.
On 17 June 2015, the Service Biosafety and Biotechnology of Sciensano organised a meeting, convening competent authorities to discuss the policy of containment of polioviruses in Belgium in view of the GAP III requirements. The identification of roles and responsibilities of parties and authorities (national, regional and international) that will be involved in the implementation and monitoring of certification against GAP III in Belgium were discussed.
In 2017, the NAC in Belgium was assigned to the International Relations and Public Health Emergencies Department of the Federal Public Service Health, Food chain safety and environment (IBRI).
In 2019, the GAPIII was legally implemented in Belgium by the Law of April 7, 2019 on prophylactic measures in the field of polio and the Royal Decree of December 11, 2019 establishing the procedures for handling and storing type 2 polioviruses. The Royal Decree also defines the certification and approval procedures for facilities that will continue working with type 2 polioviruses.
All information is available on this website.
Glossary – polioviruses containement
Facility
Any laboratory or vaccine production unit owned or operated by any level of government, academic institution, corporation, company, partnership, society, association, firm, sole proprietorship or other legal entity.
A facility designated by the ministry of health or another designated national body or authority as serving critical national or international functions that involve the handling and storage of needed poliovirus infectious materials or potentially infectious materials under conditions set out in this standard.
Any facility that is likely to investigate new WPV2, type 2 attenuated vaccine-derived poliovirus (aVDPV2), type 2 circulating vaccine-derived poliovirus (cVDPV2), or inactivated type 2 vaccine-derived poliovirus (VDPV2) isolates, or new fecal or respiratory samples originating from recent OPV-using countries, and adopts and implements 1) safe and secure working practices based on a risk assessment and the implementation of appropriate biorisk management systems as described in GAPIII, 2) a nonretention policy for WPV2 materials as of the beginning of Phase IIa of the poliovirus type 2 containment period, and 3) a nonretention policy for OPV2/Sabin2 materials as of the beginning of Phase IIb of the poliovirus type 2 containment period.
Sabin Poliovirus (OPV/Sabin strains)
Attenuated poliovirus strains (approved for use in oral polio vaccines by national regulatory authorities, principally Sabin strains).
OPV/Sabin infectious materials
These include
- cell culture isolates and reference OPV/Sabin strains;
- seed stocks and live virus materials from OPV production;
- environmental sewage or water samples that have tested positive for the presence of OPV/Sabin strains;
- faecal or respiratory secretion samples from recent OPV recipients;
- infected animals or samples from such animals, including poliovirus receptor transgenic mice;
- derivatives produced in the laboratory that have capsid sequences from OPV/Sabin strains;
- full-length RNA or cDNA that includes capsid sequences derived from OPV/Sabin strains;
- cells persistently infected with poliovirus strains whose capsid sequences are derived from OPV/Sabin strains.
OPV/Sabin potentially infectious materials
These include
- faecal or respiratory secretion samples collected for any purpose in a time and geographic area of OPV use;
- products of such materials from poliovirus permissive cells or animals;
- respiratory and enteric virus stocks handled under conditions where OPV/Sabin strain contamination or replication is possible.
VDPV
Vaccine-derived polioviruses (VDPV) are classified with wild polioviruses and usually demonstrate 1–15% sequencedifferences from the parental oral polio vaccine (OPV) strain. Some isolates display >15% sequence diversity but are phylogenetically related to parental Sabin strains. VDPV may have circulated in the community (cVDPV) or have replicated for prolonged periods in immunodeficient subjects (iVDPV) or be ambiguous and of unknown origin (aVDPV).
VAPP
Vaccine associated paralytic poliomyelitis
WPV: Wild Poliovirus
Wild polioviruses are naturally occurring isolates known or believed to have circulated persistently in the community. Vaccine-derived polioviruses (VDPV) are classified with wild polioviruses. Attenuated strains not licensed for use as live vaccines (Cox/Lederle and Koprowski/Wistar series) are classified with wild polioviruses as their clinical properties are unproven.
WPV infectious materials
These include
- clinical materials from confirmed wild poliovirus (including VDPV) infections;
- environmental sewage or water samples that have tested positive for the presence of wild polioviruses;
- cell culture isolates and reference strains of wild poliovirus;
- seed stocks and infectious materials from IPV production;
- infected animals or samples from such animals, including human poliovirus receptor transgenic mice;
- derivatives produced in the laboratory that have capsid sequences from wild polioviruses, unless demonstrably proven to be safer than Sabin strains. The safety of new derivatives containing wild poliovirus capsid sequences will be assessed by an expert panel, on the basis of comparison to reference Sabin strains for (i) degree and stability of attenuation; (ii) potential for person-to-person transmission; and (iii) neurovirulence in animal models;
- full-length RNA or cDNA that includes capsid sequences derived from wild poliovirus, unless viruses derived from them are demonstrably proven to be safer than Sabin strains. The safety of full-length RNA or cDNA containing wild poliovirus capsid sequences will be assessed by an expert panel convened by WHO, on the basis of comparison to reference Sabin strains for (i) degree and stability of attenuation; (ii) potential for person-to-person transmission; and (iii) neurovirulence in animal models;
- cells persistently infected with poliovirus strains whose capsid sequences are derived from wild poliovirus.
Wild poliovirus potentially infectious materials
These include
- faecal or respiratory secretion samples collected for any purpose in a time and geographic area of wild poliovirus (including VDPV) circulation;
- products of such materials from poliovirus permissive cells or animals;
- uncharacterized enterovirus-like cell culture isolates from countries known or suspected to have circulating wild poliovirus or VDPV at the time of collection;
- respiratory and enteric virus stocks handled under conditions where poliovirus contamination or replication is possible.